Tasty&Healthy was tested in a set of prospective clinical trials in children and young adults led by Professor Dan Turner from Jerusalem, conducted in ~20 medical
centers worldwide.

-
The Study
The TASTI-MM randomized controlled study
Read more Read less -
The "TASTI-E" randomized controlled study
Read more Read less -
The "MyTasty” study
Read more Read less -
Imaging Assessments in the Tasty & Healthy Study
Read more Read less -
Metabolomic Analysis - The TASTI-MM randomized controlled study
Read more Read less

References
-
1
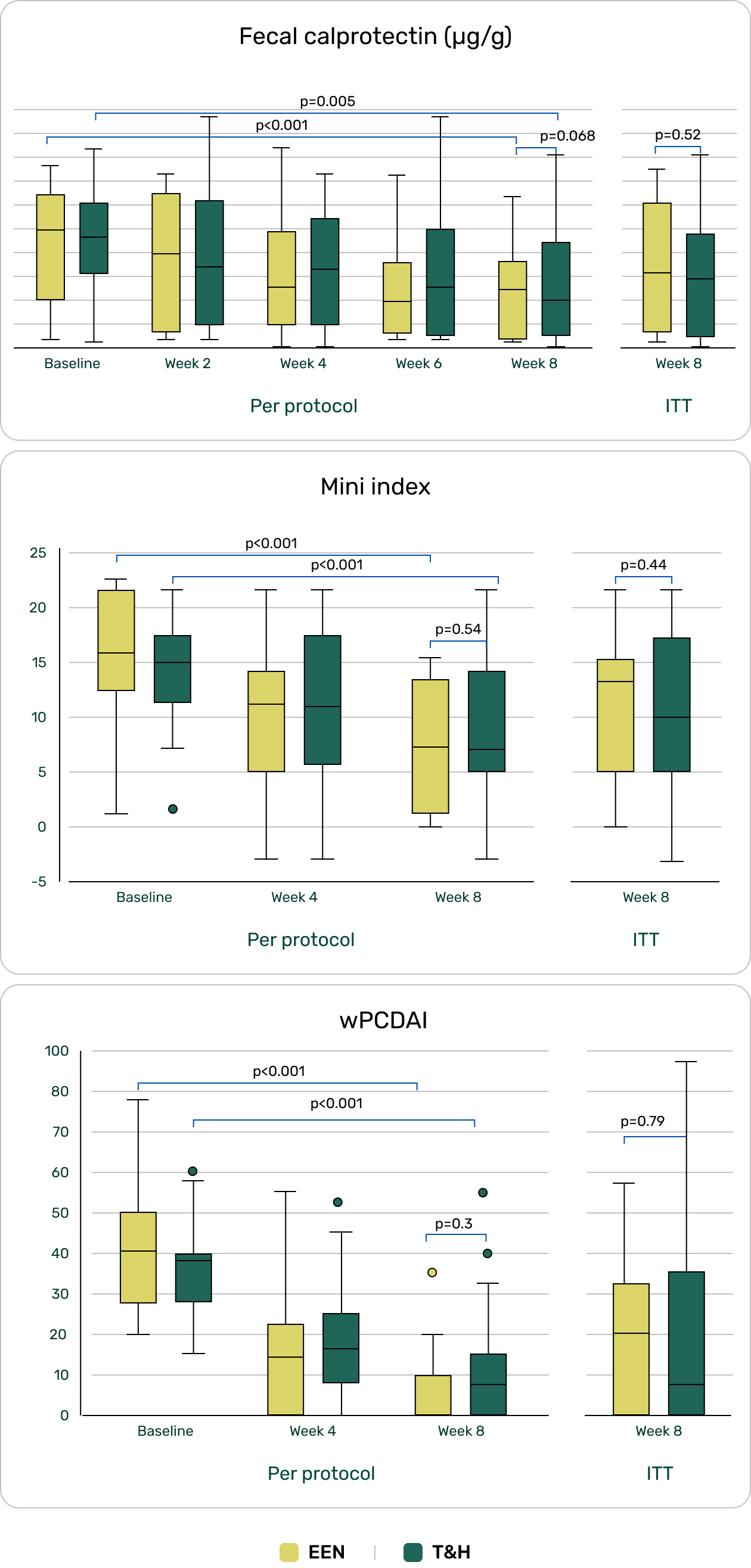
Frutkoff YA, Plotkin L, Pollak D, Livovsky J, Focht G, Lev-Tzion R, Ledder O, Assa A, Yogev D, Orlanski-Meyer E, Broide E, Kierkuś J, Kang B, Weiss B, Aloi M, Schwerd T, Shouval DS, Bramuzzo M, Griffiths AM, Yassour M, Turner D. Whole food diet induces remission in children and young adults with mild-moderate Crohn's disease and is more tolerable than exclusive enteral nutrition: a randomized controlled trial. Gastroenterology. 2025 Jun 17:S0016-5085(25)00896-0. doi: 10.1053/j.gastro.2025.06.011. Epub ahead of print. PMID: 40553742.
-
2
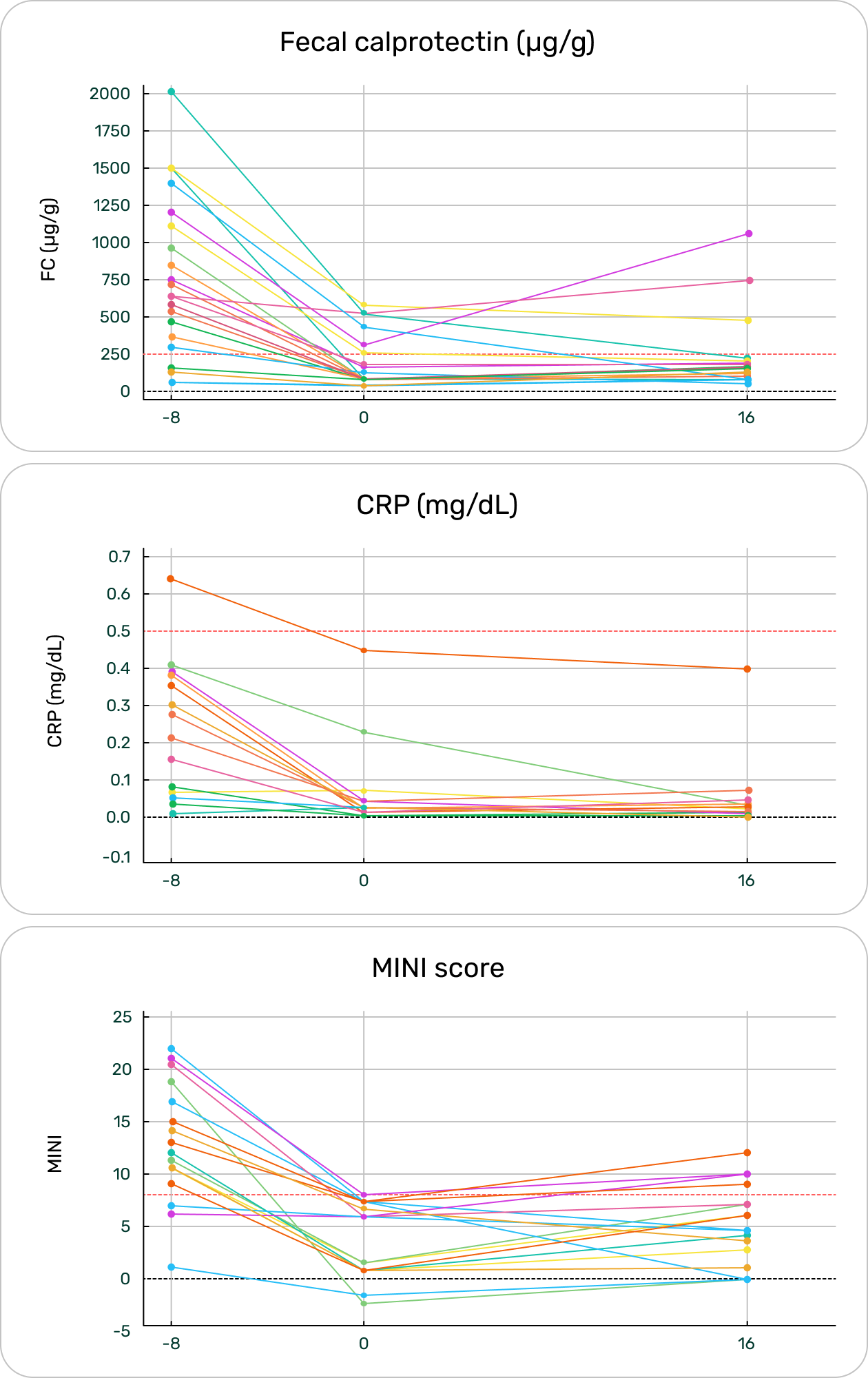
L. Plotkin, Y. Aharoni Frutkoff, Z. Shavit, G. Focht, J. Livovsky, R. Buchuk, R. Lev-Zion, R. Sigall Boneh, B. Weiss, M. Slae, I. Dotan, L. Godny, J. Kierkuś, M. Matuszczyk, E. Broide, G. Moshe, A. Griffiths, I. Martincevic, T. Naftali, L. Abramas, M. Aloi, R. Mercurio, A. Yerushalmy-Feler, T. Schwerd, E. Crowley, R. Reifen, D. Turner. Fecal calprotectin response to Tasty&Healthy dietary intervention in asymptomatic children and young adults with biologically active Crohn’s disease: results of the “TASTI-E” randomized controlled trial (P1059). J Crohn Colitis 2025; 19 (S1):i1952
-
3
L. Plotkin, Y. Aharoni Frutkoff, Z. Shavit, G. Focht, J. Livovsky, R. Buchuk, R. Lev-Zion, O. Ledder, A. Assa, R. Sigall Boneh, B. Weiss, M. Slae, I. Dotan, J. Kierkuś, E. Broide, A. Griffiths, T. Naftali, M. Aloi, A. Yerushalmy-Feler, T. Schwerd, R. Reifen, D. Turner. Personalized Tasty&Healthy whole-food diet for maintaining remission in children and adults with Crohn’s disease: results from the MyTasty open-label trial (P1093). J Crohn Colitis 2025; 19 (S1):i2009


Your Guide to a Tasty & Healthy Life!